Tannins: fascinating but sometimes dangerous molecules

Welcome to the Tannin webpage. We offer a variety of information on tannins including, but not limited to, their biosynthesis, chemical structures, toxicology, positive effects, chemical analysis....
Tannins are naturally occurring plant polyphenols. Their
main characteristic is that they bind and precipitate proteins.
They can have a large influence on the nutritive value of many
foods eaten by humans and feedstuff eaten by
animals. Tannins
are common in fruits (grapes, persimmon, blueberry, etc.), in tea,
in chocolate, in  legume forages (trefoil, etc.), in legume trees (Acacia
spp., Sesbania spp., etc.), in grasses (sorghum, corn, etc.).
legume forages (trefoil, etc.), in legume trees (Acacia
spp., Sesbania spp., etc.), in grasses (sorghum, corn, etc.).
Tannins contribute to many aspects of our daily lives. They are responsible for the astringent taste we experience when we partake of wine or unripe fruits, and for the enchanting colors seen in flowers and in autumn leaves.
For more information on tannins explore the following topics:
Definition

The word tannin is very old and reflects a traditional technology. "Tanning" (waterproofing and preserving) was the word used to describe the process of transforming animal hides into leather by using plant extracts from different plant parts of different plant species.
- Plant parts containing tannins include bark, wood, fruit, fruitpods, leaves, roots, and plant galls.
- Examples of plant species used to obtain tannins for tanning purposes are wattle (Acacia sp.), oak (Quercus sp.), eucalyptus (Eucalyptus sp.), birch (Betula sp.), willow (Salix caprea), pine (Pinus sp.), quebracho (Scinopsis balansae) .
Tannins are phenolic compounds that precipitate proteins. They are composed of a very diverse group of oligomers and polymers. There is some confusion about the terminology used to identify or classify a substance as a tannin, In fact,
- not only tannins bind and precipitate proteins (other phenolics such as pyrogallol and resorcinol also have this property),
- not all polyphenols precipitate proteins or complex with polysaccharides.
One of the most satisfactory definition of tannins was given by Horvath (1981):
"Any phenolic compound of sufficiently high molecular weight containing sufficient hydroxyls and other suitable groups (i.e. carboxyls) to form effectively strong complexes with protein and other macromolecules under the particular environmental conditions being studied"
Tannins can complex with:
- Proteins
- Starch
- Cellulose
- Minerals
Occurrence

Tannins are widely distributed in the plant kingdom. They are common both in Gymnosperms and Angiosperms. Within Angiosperms, tannins are more common in Dicotyledons than in Monocotyledons.
Examples of families of Dicotyledons rich in tannins are:
- Leguminosae : Acacia sp. (wattle); Sesbania sp.; Lotus sp. (trefoil); Onobrychis sp. (sainfoin);
- Anacardiaceae: Scinopsis balansae (quebracho)
- Combretaceae: myrobalan
- Rhizophoraceae : mangrove
- Myrtaceae: Eucalyptus sp., Mirtus sp. (Myrtle)
- Polinaceae: canaigre.
Other important tannin containing plants are Quercus sp. (oak), Acer sp. (maple), Betula sp. (birch), Salix caprea (willow), Pinus sp. (Pine), Sorghum sp.
Tannins are located mainly in the vacuoles or surface wax of the plants. In these sites they do not interfere with plant metabolism. Only after cell breakdown and death can they act and have metabolic effects.
Location of the tannins in various plant tissues:
- Bud tissues - most common in the outer part of the bud, probably as protection against freezing
- Leaf tissues - most common in the upper epidermis. However, in evergreen plants, tannins are evenly distributed in all leaf tissues. They serve to reduce palatability and, thus, protect against predators.
- Root tissues - most common in the hypodermis (just below the suberized epidermis). They probably act as a chemical barrier to penetration and colonization of roots by plant pathogens.
- Seed tissues - located mainly in a layer between the outer integument and the aleurone layer. They have been associated with the maintenance of plant dormancy, and have allelopathic and bactericidal properties.
- Stem tissues - often found in the active growth areas of the trees, such as the secondary phloem and xylem and the layer between epidermis and cortex. Tannins may have a role in the growth regulation of these tissues. They are also found in the heartwood of conifers and may be a contribute to the natural durability of wood by inhibiting microbial activity.
Biosynthesis
There are three large classes of secondary metabolites in plants:
- Nitrogen containing compounds
- Terpenoids
- Phenolics
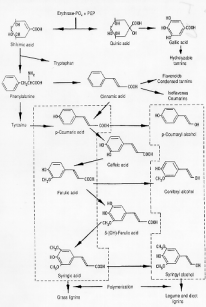
Tannins belong to the phenolics class. All phenolic compounds (primary and secondary) are, in one way or another, formed via the shikimic acid pathway, also known as the phenylpropanoid pathway.
The same pathway leads to the formation of other phenolics such as isoflavones, coumarins, lignins and aromatic aminoacids (tryptophan, phenylalanine and tyrosine).
The two main categories of tannins that impact animal nutrition are hydrolyzable tannins (Hts) and condensed tannins identified more correctly as proanthocyyanidins (Pas) that are resistant to hydrolytic degragation. An example of how several common tannins are formed is as follows:
- Gallic acid is derived from quinic acid.
- Ellagotannins are formed from hexahydroxydiphenic acid esters by the oxidative coupling of neighboring gallic acid units attached to a D-glucose core.
- Further oxidative coupling forms the hydrolyzable tannin (HT) polymers.
- Proanthocyanidin (PA) biosynthetic precursors are the leucocyanidins
(flavan-3,4-diol and flavan-4-ol)
- Upon autoxidation, in the absence of heat, they form anthocyanidin and 3-deoxyanthocianidin, which, in turn, polymerize to form PAs.
Chemical Structure
Tannins are one of the many types of secondary compounds found
in plants
Characteristics of tannins:
- oligomeric compounds with multiple structure units with free phenolic groups,
- molecular weight ranging from 500 to >20,000,
- soluble in water, with exception of some high molecular weight structures,
- ability to bind proteins and form insoluble or soluble tannin-protein complexes.
Tannins are usually subdivided into two groups:
- Hydrolyzable tannins (HT)
- Proanthocyanidins (PA) (often called Condensed Tannins)
Hydrolyzable tannins
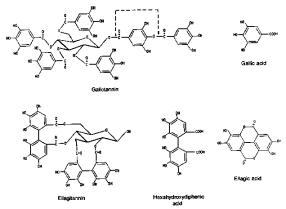
HTs are molecules with a polyol (generally D-glucose) as a central core. The hydroxyl groups of these carbohydrates are partially or totally esterified with phenolic groups like gallic acid (-->gallotannins) or ellagic acid (--> ellagitannins). HT are usually present in low amounts in plants.
Some authors define two additional classes of hydrolyzable tannins: taragallotannins(gallic acid and quinic acid as the core) and caffetannins (caffeic acid and quinic acid)
1. Gallotannins:
- The phenolic groups that esterify with the core are sometimes constituted by dimers or higher oligomers of gallic acid (each single monomer is called galloyl)
- Each HT molecule is usually composed of a core of D-glucose and 6 to 9 galloyl groups
- In nature, there is abundance of mono and di-galloyl esters of glucose (MW about 900). They are not considered to be tannins. At least 3 hydroxyl groups of the glucose must be esterified to exhibit a sufficiently strong binding capacity to be classified as a tannin.
- The most famous source of gallotannins is tannic acid obtained from the twig galls of Rhus semialata. It has a penta galloyl-D-glucose core and five more units of galloyl linked to one of the galloyl of the core.
2. Ellagitannins:
- The phenolic groups consist of hexahydroxydiphenic acid, which spontaneously dehydrates to the lactone form, ellagic acid.
- Molecular weight range: 2000-5000.
HT properties:
- hydrolyzed by mild acids or mild bases to yield carbohydrate and phenolic acids
- Under the same conditions, proanthocyanidins (condensed tannins) do not hydrolyze.
- HTs are also hydrolyzed by hot water or enzymes (i.e. tannase).
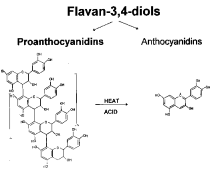
Proanthocyanidins (condensed tannins)
PAs are more widely distributed than HTs. They are oligomers or polymers of flavonoid units (i.e. flavan-3-ol) linked by carbon-carbon bonds not susceptible to cleavage by hydrolysis.
- PAs are more often called condensed tannins due to their condensed chemical structure. However, HTs also undergo condensation reaction. The term, condensed tannins, is therefore potentially confusing.
- The term, proanthocyanidins, is derived from the acid catalyzed oxidation reaction that produces red anthocyanidins upon heating PAs in acidic alcohol solutions.
- The most common anthocyanidins produced are cyanidin (flavan-3-ol, from procyanidin) and delphinidin (from prodelphinidin)
- PAs may contain from 2 to 50 or greater flavonoid units; PA polymers have complex structures because the flavonoid units can differ for some substituents and because of the variable sites for interflavan bonds.
- Anthocyanidin pigments are responsible for the wide array of pink, scarlet, red, mauve, violet, and blue colors in flowers, leaves, fruits, fruit juices, and wines. They are also responsible for the astringent taste of fruit and wines.
- PA carbon-carbon bonds are not cleaved by hydrolysis.
- Depending on their chemical structure and degree of polymerization, PAs may or may not be soluble in aqueous organic solvents.
Interaction with other Macromolecules
Tannins have a major impact on animal nutrition because of their ability to form complexes with numerous types of molecules, including, but not limited to,
- Carbohydrates,
- Proteins,
- Polysaccharides,
- Bacterial cell membranes,
- Enzymes involved in protein and carbohydrates digestion.
Carbohydrates
Both starch and cellulose are complexed by tannins (especially by PAs):
- Starch-tannin interaction - starch has the ability to form hydrophobic cavities that allow inclusion complexes with tannins and many other lipophyllic molecules. Only starch, among the molecules that are bound by tannins, has this embedding characteristic.
- Cellulose-tannin interaction - cellulose has a direct surface interaction with tannins.
- Cell wall carbohydrate-tannin interaction - this association is less understood. One explanation is that tannins associate with plant cell walls in a manner reminiscent to that of lignin. However, another explanation is that this association is merely an artifact of tannin isolation from non-living cells. Indeed, the location of tannins and cell wall carbohydrates is quite different in living cells than in plant cells after digestion by animals.
- Tannin-carbohydrate interactions are increased by carbohydrates with high molecular weight, low solubility and conformational flexibility. These interactions are probably based on hydrophobic and hydrogen linkages.
Proteins
The capacity of tannins to bind proteins has been recognized for centuries. Leather tanning is a very ancient practice. Tannin-protein interactions are specific and depend on the structure of both the protein and tannin.
- Protein characteristics that favor strong bonding
- large molecular size,
- open and flexible structures,
- richness in proline.
- Tannin characteristics that favor strong bonding
- high molecular weight,
- high conformational mobility.
Chemical linkages
Tannin-protein interactions are most frequently based on hydrophobic and hydrogen bonding. Ionic and covalent bonding occur less frequently.
- The tannin's phenolic group is an excellent hydrogen donor
that forms strong hydrogen bonds with the protein's carboxyl
group.
- For this reason, tannins have a greater affinity to proteins than to starch.
- Hydrophobic bonds are stronger at higher ionic strength (higher tannin/protein ratios) and higher temperatures.
- Covalent bonding occurs only under oxidizing conditions
such as
- autoxidation over time, or
- action of oxidative enzymes (i.e. polyphenoloxydases and peroxidases). Covalent bonding is far more difficult to disrupt than the previous types of bonding and is nutritionally very important because of its irreversible nature.
- Precipitation of proteins by tannins is maximum at
pH values near the isoelectric point of the protein.
- In solution at high pH, phenolic hydroxyls are ionized and proteins have net negative charges. Under these conditions, precipitation does not occur because proteins exhibit repulsive forces.
- Strong complexes with tannins are formed by tannin-binding agents like polyvinylpyrrolidone (PVP) and polyethylen glycol (PEG), and protein denaturants like phenol.
- To have high protein affinity, tannins must be small enough to penetrate interfibrillar region of protein molecules but large enough to crosslink peptide chains at more than one point.
- HTs and PAs form tannin-protein complexes in similar manners. Proteins thus bound are generally resistant to attack by proteases and hence may be unavailable for livestock nutrition. However, it is hypothesized that HTs may have a less damaging effect on protein digestion because these tannins may hydrolyze in the acidic gastric environment and release the bound proteins.
- When soluble tannins interact with proteins, both soluble
and insoluble complexes are formed; their relative proportion
depends on the concentration and size of both molecules.
- Soluble complexes are favored when protein concentration is in excess (fewer tannin attachment sites per each protein molecule). Soluble complexes represent an analytical problem because they do not precipitate and, thus, are difficult to measure.
- Insoluble complexes are formed when tannins are present in excess and form an hydrophobic outer layer in the complex surface.
Nutritional Effects: toxic and antinutritional effects
Tannins act as a defense mechanism in plants against pathogens, herbivores and hostile environmental conditions. Generally, tannins induce a negative response when consumed. These effects can be instantaneous like astrigency or a bitter or unpleasant taste or can have a delayed response related to antinutritional/toxic effects.
This section will cover the effect of tannins on:
Intake, Feed digestibility, Toxicity to microorganisms, Toxicity to ruminants, Toxicity to monogastric, Animals' defense mechanisms
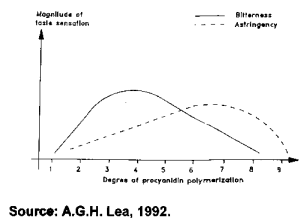
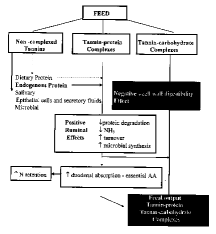
Tannins negatively affect an animal's feed intake, feed digestibility, and efficiency of production. These effects vary depending on the content and type of tannin ingested and on the animal's tolerance, which in turn is dependent on characteristics such as type of digestive tract, feeding behavior, body size, and detoxification mechanisms.
Sites of action of tannins :
- Oral cavity - mastication ruptures the plant cell tissue and exposes proteins and carbohydrates to tannins.
- Rumen and gastrointestinal tract lumen - unbound tannins complex dietary proteins and metabolic proteins (e.g. bacteria, enzymes, epithelial cells).
Tannins may reduce intake by decreasing palatability and by negatively affecting digestion.
- Palatability is reduced because tannins are astringent.
Astringency is the sensation caused by the formation of
complexes between tannins and salivary glycoproteins.
- Low palatability depresses feed intake and, thus, animal productivity.
- Digestibility reduction (see following section) negatively influences intake because of the filling effect associated with undigested feedstuff.
- Several studies have reported higher feed intakes and weight
gains when tannin-free diets were compared to tannin-containing
ones.
- Some caution must be taken when interpreting these results. In many trials, commercial tannins sources were used. These types of tannins are usually more effective at lowering feed intakes than naturally-occurring tannins.
- Another likely problem in many trials is that often only extractable tannins are measured and insoluble tannins are not quantified. However, insoluble tannins may have equal or greater biological activity than those that are more easily extracted.
- When naturally-occurring tannins are used, these tannins do not always reduce intake. In some trials, tannin-rich diets were eaten in equal or larger amounts than low or free tannin diets.
- The form in which the forage is fed may influence how tannins affect feed intake. Forages rich in tannins are eaten in larger amounts when field dried rather than fresh frozen. Indeed, drying reduces the solubility of tannins and, hence, reduces their ability to complex proteins (tannins become more polimerized, resulting in a lower number of free hydroxyls available for binding the proteins).
- Intake in animal diets rich in tannins can be increased by
using a compound with a high affinity for tannins, like PEG (polyethylene
glycol).
- PEG has a higher affinity to tannins than do proteins.
- PEG can be sprayed on the forages or added in the diet and is fairly inexpensive.
- PEG utilization can increase feed palatability and digestibility and result in higher animal productivity.
- Feed intake may also be decreased by low molecular weight phenolics. They predominate during the early stages of plant growth and are then converted to oligomers and finally to polymers (tannins) when the plant mature. These low MW phenolics are absorbed into the body and exhibit systemic effects such as alteration of physiological systems, increased energy requirements due to detoxification, and subsequent growth rate reduction.
Usually PAs are not absorbed through the digestive tract. Instead, free tannins and complexed forms remain in the rumen, decreasing protein and plant cell wall digestibility.
Carbohydrate digestibility
- Several studies have shown that tannins decrease organic matter and fiber digestion.
- The lower digestibility is the result of the interaction of tannins with cellulase enzymes and rumen bacteria.
- In some cases, lower fiber digestibility can be the result of a shortage of ruminally fermented nitrogen due to the complexation of proteins by tannins.
- Field drying and treatments with PEG are able to limit these negative effects.
- In some cases, lower digestibility was compensated by higher protein outflow from the rumen.
Protein digestibility
- In in vivo studies, protein digestibility is greatly reduced when tanniniferous feeds are part of the diet.
- Plants high in PAs often have proteins linked tightly to the
plant cell wall (neutral-detergent insoluble nitrogen, NDIN) and
lignin (acid detergent lignin, ADL) components, and, thus, may
show negative digestion coefficients when ingested.
- After ingestion, PAs may also form detergent insoluble tannin-protein complexes with proteins they encounter. These two factors may cause the amount of NDIN and ADL excreted in the feces to exceedthe amount ingested.
- When dietary content of tannins increases, fecal nitrogen excretion increases due to lower digestibility of nitrogen fractions and formation of tannin-protein complexes.
- However, despite the decrease in apparent nitrogen digestibility, nitrogen retention does not always decrease with increasing tannin concentration in the diet. In many cases, nitrogen retention increases as a result of decreased urinary excretion.
- The effect of tannins on protein digestibility of a specific
feed can be estimated using Lucas's test (Van Soest, 1994, page
360).
- If protein digestibility is not affected by tannins, proteins behave as a uniform fraction, with a regression coefficient (true digestibility) equal to or larger than 0.88, with a negative intercept(estimate of metabolic endogenous nitrogen, usually about 0.5% of dry matter intake or smaller) and with a low standard error.
- However, if protein digestibility is affected by tannins, the proteins will behave as a non-uniform fraction, with a regression coefficient (true digestibility) smaller than 0.88, and with a larger negative intercept and a higher standard error
- Tannin solubility plays a role in determining a tannin's
efficiency in binding proteins and/or fiber.
- If the ratio of soluble to insoluble tannins is high, then protein digestibility is affected more than fiber digestibility.
- If the same ratio is low, fiber digestibility is the most affected.
Tannin toxicity to rumen microorganisms has been described for several bacteria species such as Streptococcus bovis, Butyvibrio fibrosolvens, Fibrobacter succinogenes, Prevotella ruminicola, and Ruminobacter amylophilis.
- Three mechanisms of toxicity have been identified
- enzyme inhibition and substrate deprivation,
- action on membranes,
- metal ion deprivation.
- Tannins induce changes in morphology of several species of ruminal bacteria.
- Microrganism defense mechanisms involve
- secretion of binding polymers,
- synthesis of tannin-resistant enzymes,
- biodegradation of tannins (peculiarity of some recently discovered bacteria that are able to tolerate high levels of PA).
Hydrolizable tannins
Hydrolizable tannins are toxic to ruminants. Tannin toxicity from HTs may occur in animals fed oak (Quercus spp.) and several tropical tree legumes (e.g. Terminalia oblongata and Clidema hirta)
Microbial metabolism and gastric digestion convert HTs into absorbable low molecular weight metabolites. Some of these compounds are toxic.
- The major lesions associated with HT poisoning are hemorrhagic gastroenteritis, necrosis of the liver, and kidney damage with proximal tuberal necrosis,
- High mortality and morbidity were observed in sheep and cattle fed oaks and other tree species with more than 20% HT.
Protanthocyanidins
Toxicity from PA is difficult to separate from their effects on the digestion of proteins and carbohydrates.
- PAs are not absorbed by the digestive tract,
- PAs may damage the mucosa of the gastrointestinal tract, decreasing the absorption of nutrients,
- PAs may reduce the absorption of essential aminoacids. The most susceptible amino acids are methionine and lysine.
- Decreased methionine availability could increase the toxicity of cyanogenic glycosides, because methionine is involved in the detoxification of cyanide via methylation to thiocyanate.
Animals fed diets with a level of tannins under 5% experience
- depressed growth rates,
- low protein utilization,
- damage to the mucosal lining of the digestive tract,
- alteration in the excretion of certain cations, and
- increased excretion of proteins and essential amino acids.
In poultry, small quantities of tannins in the diet cause adverse effects
- levels from 0.5 to 2.0% can cause depression in growth and egg production,
- levels from 3 to 7% can cause death.
In swine, similar harmful effects of tannins have been found.
The addition of additional proteins or amino acids may alleviate the antinutritional effects of tannins.
Levels of tannins above 5% of the diet are often lethal.

Hoatzin: a ruminant-like bird that eats a lot of tannin-rich leaves
Some insects consume leaves with high levels of tannins. They are able to adapt to tannins using several available mechanisms
- alkaline gut pH,
- presence of surfactants to decrease affinity between ingested tannins and protein,
- presence of peritrophic membranes that absorb tannins and are then excreted in the feces.
Many tannin-consuming animals secrete a tannin-binding protein (mucin) in their saliva.
- Tannin-binding capacity of salivary mucin is directly related
to its proline content. Advantages in using salivary proline-rich
proteins (PRPs) to inactivate tannins are
- PRPs inactivate tannins to a greater extent than do dietary proteins; this results in reduced fecal nitrogen losses,
- PRPs contain non specific nitrogen and nonessential amino acids; this makes them more convenient for an animal to exploit rather than using up valuable dietary protein.
- There are species differences in the amount of PRP that different
species produce to bind tannins
- Ability to tolerate tannins - deer> goat> sheep> cattle
- Consumption of high tannin diets stimulates the development of the salivary glands to permit more PRP production,
- Some researchers claim that sheep and cattle do not have any PRPs.
Nutritional Effects: positive effects
The presence of tannins in food sources for monogastric animals, is generally viewed adversely, though their contribution to red wines is certainly an exception. However, in ruminants, tannins can induce beneficial effects. For example,
- In sheep and cattle higher retention of nitrogen has
been observed in sheep and cattle with low to moderate levels
of tannins in forages,
- In these cases, the lower apparent and true digestibility of nitrogen was compensated for by reduced urinary loss of hydrogen,
- Moderate levels of tannins (less than 4% ) in forage legumes can have beneficial responses in ruminants, resulting in higher growth rates and milk yield,
- However, even in ruminants, levels of tannins exceeding 6% of the diet result in negatively affect growth rates and milk yield.
Several mechanisms have been suggested to explain how tannins influence protein utilization by ruminants -
Rumen escape
One mechanism postulated is that tannins complex proteins at the pH of the rumen (5 to 7) and protect them from microbial enzymes. Subsequently, these complexes dissociate in contact with gastric (pH 2.5-3.5) and pancreatic (pH 8) secretions.
- High quality dietary proteins would be protected, at least
in part, from degradation in the rumen and would then be digested
more effectively in the intestine. However,
- Even when released, tannins are still biologically active and can react with digestive enzymes or other proteins.
- Indeed, in nonruminants, tannins decrease intestinal absorption of amino acids (especially methionine) and reduce growth.
- Tannin-protein complexes that are strong enough to survive the environment of the rumen may not be broken down and digested in the lower tract.
Urea recycling
Another hypothesis is that tannins may increase efficiency in
nitrogen recycling to the rumen.
Some facts -
- Tannins lower the rate of protein degradation and deamination
in the rumen resulting in lower rumen ammonia concentration.
- This results in lower plasma urea nitrogen (PUN).
- Lower PUN means lower urinary nitrogen excretion with less wastage of nitrogen.
- Larger amounts of nitrogen are recycled because tannins stimulate increased saliva production.
Microbial efficiency
In diets based on tanniniferous forages, nitrogen rumen outflow is often larger than nitrogen intake. Several studies have reported an increase in protein flow when moderate doses of tannins were used. This has been attributed to -
- increased rumen escape of dietary proteins,
- increase in microbial protein flow (up to 28% in sheep).
The larger microbial flow could be the result of
- Increased saliva production, increased rumen turnover rate, and hence, increased microbial outflow,
- Increased nitrogen recycling to the rumen,
- Decreased proteolysis and slower fermentation of proteins and non-protein nitrogen in the rumen (particularly important in legume silages); this results in a more even nitrogen availability to bacteria.
Microbial flow is usually measured using a microbial internal marker (diaminopimelic acid, DAPA). However, tannins may reduce the extraction of microbial cells walls from digesta and make microbial flows measured with DAPA unreliable
Chemical Analysis
The amount and type of tannins synthesized by plants varies considerably depending on plant species, cultivars, tissues, stage of development, and environmental conditions. Therefore, the study of the nutritional effects of tannins on animals requires quantification of the tannins present in a particular diet.. Due to the complexity of tannins, several methods have been developed for their quantification. None of them, however, is completely satisfactory.
Sample preparation
The first factor to consider is how the forage or the feed is consumed by the animal - feeds should be analyzed in the form eaten by the animals
If the samples are collected fresh and they have to be stored, freeze-drying is the gentlest method of preservation and is recommended instead of freezing, air or oven-drying.
- If freeze drying is too expensive or the equipment is not available, freezing without thawing of the sample before extraction is suggested.
- If drying is the only means available for preserving the material, drying temperature should be higher than 40° C (to avoid oxidation by the still active enzymes) and lower than 60° C (to avoid heat damage and polymerization).
Sample handling
After cutting the sample should be -
- Stored in a cold, dark container,
- Cut in small pieces and freeze with liquid nitrogen,
- Pulverized with a mortar and a pestle, and
- Immediately extracted or freeze dried and stored at -4° C.
Sample extraction
Tannins are extracted with an aqueous organic solvent.
- 70% acetone and 30% water is a more effective extractant
than alcoholic solvents.
- Acetone inhibits tannin-protein interaction. This is a limitation in protein precipitation assays.
- In many plants, there is a large fraction (sometimes >50%)
of the tannins that cannot be extracted (insoluble tannins).
- This unextractable fraction cannot be ignored because of its nutritional effects.
Sample purification and isolation
Tannins need to be purified from low molecular weight phenolics and pigments that are present in crude plants extracts.
- Purification is essential for the preparation of suitable standards.
- Purification of large quantities of tannins can be done by
taking advantage of their absorption by Sephadex LH-20.
Reference: Hagerman A.E., Klucher K.M., 1986 - Tannin-protein interaction. In:Plant flavanoids in biology and medicine: biochemical, pharmacological, and structure-activity relationships. Ed. Cody V., Middleton E. Jr., Harborne J. - Alan R. Liss, New York, pp 67-76. - An alternative and faster isolation method has been developed by Giner-Chavez, 1996 (see "mixed assays").
Chemical assays
Tannin assays can be divided into Colorimetric, Gravimetic, Protein precipitation, and Mixed.
A. Colorimetric assays
Folin-Dennis method and its modifications (Folin-Ciocalteau method)
- The reaction is based on the reduction of phosphomolybdic acid by phenols in aqueous alkali.
- The method determines the total free phenolic groups and is therefore a method to determine total soluble phenolics (either HT and PA).
- Problem: It does not differentiate between tannins and many phenolics that are not tannins. Interfering compounds such as ascorbic acid, tyrosine and possibly glucose are also measured.
Vanillin-HCl assay
- Specific for PAs or condensed tannins.
- Exo-type reaction - vanillin reacts with the meta-substituted A-ring of flavanols to form a chromophore; the number of flavanols is proportional to the absorbance of the solution.
- Problems:
- Low molecular weight flavanols overreact and large polymers underreact,
- Catechin is used as standard. This monomer gives the maximum optical density leading to underestimation of large polymers.
Butanol-HCl assay
- Specific for PAs or condensed tannins
- Endo-type reaction - the method involves the HCl catalyzed depolimerization of condensed tannins in butanol to yield a red anthocyanidin product that can be detected spectrophotometrically.
- Problem: Tannin polymers are cleaved into dimers or trimers instead of monomers and this leads to underestimation.
- The degree of polymerization of the PAs can be estimated by combining the butanol-HCl assay with the vanillin assay
- The acid butanol assay measures the total number of flavanoid residues present and the vanillin assay measures the number of molecules.
- The butanol-HCl assay is also used to estimate the amount of insoluble tannins from extraction residues or from NDF.
- Problem: Not all red pigments dissolve, resulting in tannin underestimation.
Rhodanine assay
- Specific to gallotannins ( one type of HT)
- The sample is subjected to hydrolysis to release gallic acid. The reaction between gallic acid and the dye rhodanine produces an intense color that is measured spectrophotometrically.
Wilson and Hagerman assay
- Specific for ellagitannins ( another HT)
- The sample is subjected to hydrolysis to release ellagic acid. The reaction between ellagic acid and the sodium nitrite produce a colored solution that is measured spectrophotometrically.
All the above mentioned colorimetric methods are described in:Waterman P.G., Mole S. (1994) - Analysis of phenolic plant metabolites. Blackwell Scientific Publications, Oxford, UK
B. Gravimetric assays
Gravimetric method with Ytterbium (Reed et al., 1985)
- Determines only soluble tannins present in plant extracts; insoluble tannins are not measured.
- Based on the ability of trivalent ytterbium to selectively precipitate polyphenols from plant extracts.
- Advantages:
- Standards are not needed,
- The precipitate can be easily dissolved with oxalic acid to yield a solution of polyphenolics and insoluble Yb-oxalate. The solution can be used for further analysis (colorimetric analysis, chromatography, inhibition studies).
- Problems:
- Not all polyphenols are precipitated.
- Low repeatability in plants with low levels of tannins.
Reference: Reed J.D., Horvath P.J., Allen M.S., Van Soest P.J. (1985) - Gravimetric determination of soluble phenolics including tannins from leaves by precipitation with trivalent ytterbium. J. Sci. Food Agric., 36:255-261
Gravimetric method with PVP (Makkar et al., 1995)
- Determines only soluble tannins present in plant extracts; insoluble tannins are not measured.
- PVP irreversibly binds tannins.
- This method is not very sensitive and tends to underestimate tannins.
Reference: Makkar H.P.S., Blümmel M., Becker K. (1995) - Formation of complexes between polyvinil pyrrolidones or polyethylene glycols and tannins and their implication in gas production and true digestibility in in vitro techniques. Br. J. Nutrition, 73:897-913
Gravimetric method based on the detergent system (Horvarth et al., 1981)
- Includes soluble and insoluble tannins.
- Steps -
- Measurement of the acid-detergent residue of the NDF (NAD) and the neutral-detergent residue of the ADF (AND),
- The difference NAD-AND is used to estimate tannins. This value has been successfully used in the summative equation of Van Soest to estimate the fraction of the feeds that is indigestible due to the action of tannins.
- Problems: many soluble tannins are not measured.
Reference: Horvath, P.J. (1981) - The nutritional and ecological significance of acer-tannins and related polyphenols. M.S. Thesis. Cornell University, Ithaca, NY, USA
C. Protein precipitation assays
These methods are more closely related to the biological effects of tannins.
Radial diffusion assay (Hagerman, 1987)
- This method depends on the formation of complexes between tannins and bovine serum albumin embedded in agar.
- Plant extracts are placed in a well in the agar. They diffuse in the agar and precipitate the albumin if tannins are present. In this case a opaque circle forms.
- The diameter of the circle is proportional to the amount of tannins in the extract
- Suitable standards are necessary to estimate the amount of tannins.
- The most commonly used standard is tannic acid and the results are expressed in tannic acid equivalents.
- This method allows determination of large number of samples with limited laboratory facilities.
- Problem: less useful for quantification than the colorimetric procedures.
Reference: Hagerman A.E. (1987) - Radial diffusion method for determining tannins in plant extracts. J. Chem. Ecol.,13: 437-449
D. Mixed assays
Method of Giner-Chavez, 1996
This a method for condensed tannins or PAs that combines some previous methods in an attempt to eliminate their major problems and reduce the time required for the analysis.
The method consists of -
- Extraction of tannins from plant samples using 70% aqueous acetone (traditional method).
- Isolation of plant condensed tannins using trivalent ytterbium to prepare the standard.
- Analysis of the condensed tannins using the butanol-HCl method (traditional method).
The main innovation in this method is that instead of using an external standard like quebracho (as suggested for the butanol assay), internal standards (tannins from the same plant under analysis) are used.
- External standards have the serious limitation that the extinction coefficients for the chromophores produced with them usually are different from those obtained from the plant extracts
- In other words, each gram of external standard (i.e. cyanidin or quebracho) has a different absorption than each gram of tannin from the plant extract. Moreover, the absorption varies with plant species because of the wide variety of tannin types present in nature.
- In Giner-Chavez's method, the internal standard is obtained by precipitating tannins with ytterbium trivalent such as in Reed's method.
- In this way, even though not all tannins present in the plants extracts are precipitated by Yb, it is possible to isolate and quantify tannins for each plant that can then be used for the standard curve.
- The same goal could have been achieved by isolating the tannins with Sephadex (see "Sample purification") but it takes twice as much time (4 days vs. 2 days).
- The use of internal standards provides a more realistic estimation than with external standards.
- Using quebracho, the tannin content of Desmodium ovalifolium was found to be over 200% !! However, the value was about ten times smaller when an internal standard was used.
Reference: Giner-Chavez B.I. (1996) - Condensed tannins in tropical forages - Ph. D. Thesis. Cornell University, Ithaca, NY, USA
References
These pages were written by Antonello Cannas, a Ph.D. student in Animal Science, Cornell University. I am from Sardinia, Italy. I work as a researcher in the University of Sassari, Sardinia. My research focuses on dairy sheep nutrition. I have been educated to love tannins, especially when diluted with a 14% aqueous solution of alcohol and matched with aged Sardinian sheep cheese and some bread.
A large part of the text, the pictures, and the chemical structures of tannins have been taken from the Ph.D. Thesis of Bertha Iliana Giner-Chavez, whom, by the way, was my lovely officemate and friend. Her Ph. D. thesis has a very nice introduction and literature review on tannins in animal nutrition. It is the clearest review on the subject I have had a chance to read. Moreover, in her thesis she describes the method she developed to analyze tannins. She is from Mexico, a country where tannins in animal nutrition are as common as jalapeños in human nutrition.
Another very good review from which I fished a lot is the one by Jess Reed.
A great and complete source on tannins is the 13th chapter of the "bible" of animal nutrition written by Peter J. Van Soest, whom, by the way, has been my Advisor during my Master and is my co-Chair for the Ph. D.
References
Giner-Chavez B.I. (1996) - Condensed tannins in tropical forages - Ph. D. Thesis. Cornell University, Ithaca,
NY, USA.
Haslam E. (1989) - Plant polyphenols. Cambridge University Press, Cambridge, UK.
Chemistry and significance of condensed tannins (1989). Ed. by Hemingway R.W. and Karchesy J.J.. Plenum
Press, New York.
Reed, J.D. (1995) - Nutritional toxicology of tannins and related polyphenols in forage legumes. J. Anim.
Sci. 73:1516-1528.
Van Soest P.J. (1994) - Nutritional ecology of the ruminants (2nd ed.). Cornell University Press, Ithaca,
NY, USA.
Waterman P.G., Mole S. (1994) - Analysis of phenolic plant metabolites. Blackwell Scientific Publications,
Oxford, UK.
[Definition] [Occurrence] [Biosynthesis] [Chemical structure] [Interaction with other macromolecules] [Nutritional effects] [Chemical analysis] [References] [Return to list of toxicants]